The last two blogs have been devoted to hard water and water with high iron content. Both problems have something in common, and that is how they can be resolved. Both hardness and iron are media that need to be removed from your water, and the way that is done is via a water softener.What is a Water Softener? Basically, softeners utilize two tanks and operate using an ion exchange system. The ion replacement takes place in one tank filled with small polystyrene beads, or resin. The beads are negatively charged and are bonded to positively charged sodium ions. As the water flows past the beads, the sodium ions swap places with the calcium and magnesium ions, which carry a stronger positive charge.
In the other tank is salt. After several cycles, calcium and magnesium replace all of the sodium in the beads. When this happens the unit can no longer soften water. To solve this, the softener enters a regeneration cycle during which it soaks the beads in a mixture of water and salt (sodium chloride). The large amount of sodium in the brine causes the calcium, magnesium, and iron ions in the beads to give way and the beads are then recharged with sodium. After this regeneration, the softener flushes the remaining brine as well as the calcium and magnesium down through a drainpipe.
For those on a low sodium diet concerned with the amount of salt your water is flowing through, generally the amount of salt added to your water is very low- generally less than 12.5 milligrams per 8 oz. glass of water; much lower than the Drug and Food Administration’s standard for “very low sodium”, which is less than 35mg of sodium per serving. A “low sodium diet” according to the USDA is less than 1500mg/day, or 120 glasses of softened water! But if sodium is just a deal breaker, there is a company that makes pellets of potassium-chloride that can be used in place of salt.
Standard Water Softener vs. Iron Water Softener. Although we use the same water softening equipment to remove both hardness and iron, there is a slight difference between a system meant to remove just hardness and one meant to remove hardness and iron. An Iron Water Softener uses a smaller, finer resin bead than a standard water softener. This finer resin allows the softener to remove a higher level of iron than the standard resin. A standard softener should only remove a small level of iron whereas the finer resin can remove up to 10ppm of iron. Why not just use the finer resin in all softeners? Simply, it is more expensive, so let’s not spend our hard earned money unless it is necessary!
Advances in Technology A traditional water softener has a time clock head that determines when to rinse off the resin with the brine solution. Basically, a technician takes the level of hardness, asks the homeowner about water usage details, and then makes a calculated guess as to when the softener needs to enter rinse mode because the resin can no longer soften the water. But thanks to modern technology, we now have what we call metered heads.
New softener heads have a meter that measures how many gallons of water have been used during the current cycle. The head is digitally programmed with the hardness level of the water and actually calculates when the resin is full. Once the resin is full the system enters the rinse mode. This saves energy, salt, and water!
And that is everything you wanted (or didn’t want) to know about water softeners!






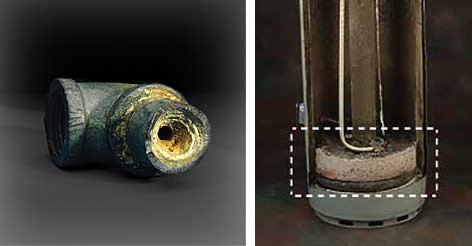 Hard water builds up and forms deposits that clog up your pipes. These deposits, of course, restrict the flow of your water and can corrode pipes, dishwashers and washing machines as well as the various pieces of plumbing equipment you have in your home. Maybe most importantly, hard water causes scale and sediment buildup in your water heater (as pictured in the second image) decreasing heating efficiency, gallon capacity, and the life expectancy of the water heater.
Hard water builds up and forms deposits that clog up your pipes. These deposits, of course, restrict the flow of your water and can corrode pipes, dishwashers and washing machines as well as the various pieces of plumbing equipment you have in your home. Maybe most importantly, hard water causes scale and sediment buildup in your water heater (as pictured in the second image) decreasing heating efficiency, gallon capacity, and the life expectancy of the water heater.

 TDS is like putting salt into water, as you add the salt it disappears into the water. Pure water would have a TDS of 0ppm. Typical well systems have a TDS anywhere from 50 to 940ppm (our office’s personal record). On average, our customers well water TDS is 150-250ppm.
TDS is like putting salt into water, as you add the salt it disappears into the water. Pure water would have a TDS of 0ppm. Typical well systems have a TDS anywhere from 50 to 940ppm (our office’s personal record). On average, our customers well water TDS is 150-250ppm.